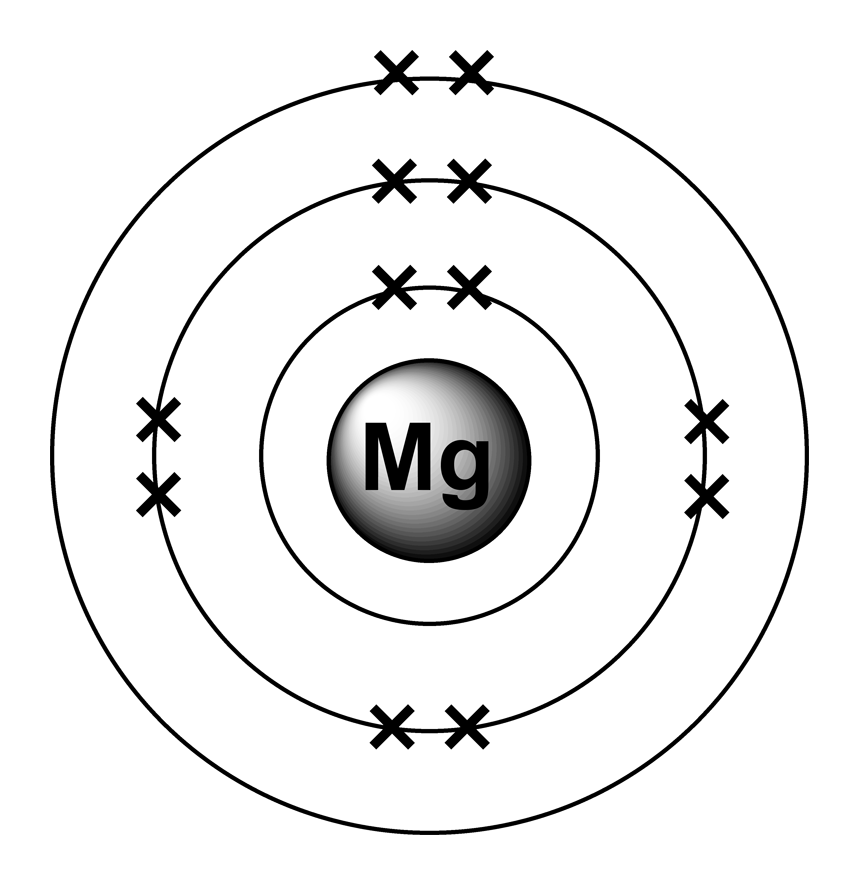
Element Magnesium Has Valence Electrons . magnesium has two valence electrons. The highest principal quantum number. An element in group 2 has two valence. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. magnesium, an alkaline earth metal, has 2 valence electrons. The oxidation state tells how many valence electrons an atom accepts (negative. Magnesium has a melting point of 648.8°c, boiling point of 1090°c, specific gravity of 1.738 (20°c), and valence of 2. The valence electrons of magnesium can be determined through its. Carbon’s ground state electron configuration is 1s 2 2s 2 2p 2. 119 rows table of oxidation states of the elements. magnesium is element 12 and belongs to group 2 of the periodic table.
from www.animalia-life.club
Carbon’s ground state electron configuration is 1s 2 2s 2 2p 2. The valence electrons of magnesium can be determined through its. magnesium, an alkaline earth metal, has 2 valence electrons. Magnesium has a melting point of 648.8°c, boiling point of 1090°c, specific gravity of 1.738 (20°c), and valence of 2. An element in group 2 has two valence. magnesium is element 12 and belongs to group 2 of the periodic table. The oxidation state tells how many valence electrons an atom accepts (negative. 119 rows table of oxidation states of the elements. The highest principal quantum number. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or.
Magnesium Electron Configuration
Element Magnesium Has Valence Electrons An element in group 2 has two valence. An element in group 2 has two valence. The oxidation state tells how many valence electrons an atom accepts (negative. magnesium has two valence electrons. magnesium, an alkaline earth metal, has 2 valence electrons. The highest principal quantum number. Magnesium has a melting point of 648.8°c, boiling point of 1090°c, specific gravity of 1.738 (20°c), and valence of 2. 119 rows table of oxidation states of the elements. The valence electrons of magnesium can be determined through its. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. magnesium is element 12 and belongs to group 2 of the periodic table. Carbon’s ground state electron configuration is 1s 2 2s 2 2p 2.
From bannerhome.weebly.com
Periodic table of elements with valence electrons bannerhome Element Magnesium Has Valence Electrons The highest principal quantum number. An element in group 2 has two valence. Carbon’s ground state electron configuration is 1s 2 2s 2 2p 2. The oxidation state tells how many valence electrons an atom accepts (negative. 119 rows table of oxidation states of the elements. magnesium, an alkaline earth metal, has 2 valence electrons. magnesium has. Element Magnesium Has Valence Electrons.
From valenceelectrons.com
Magnesium Electron Configuration Aufbau & Bohr Model Element Magnesium Has Valence Electrons 119 rows table of oxidation states of the elements. Magnesium has a melting point of 648.8°c, boiling point of 1090°c, specific gravity of 1.738 (20°c), and valence of 2. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. magnesium, an alkaline earth metal, has 2. Element Magnesium Has Valence Electrons.
From www.pinterest.co.uk
Magnesium, atomic structure Stock Image C018/3693 Science Photo Element Magnesium Has Valence Electrons The highest principal quantum number. The valence electrons of magnesium can be determined through its. magnesium has two valence electrons. Carbon’s ground state electron configuration is 1s 2 2s 2 2p 2. magnesium, an alkaline earth metal, has 2 valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which. Element Magnesium Has Valence Electrons.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Element Magnesium Has Valence Electrons magnesium has two valence electrons. The highest principal quantum number. 119 rows table of oxidation states of the elements. Magnesium has a melting point of 648.8°c, boiling point of 1090°c, specific gravity of 1.738 (20°c), and valence of 2. An element in group 2 has two valence. magnesium, an alkaline earth metal, has 2 valence electrons. . Element Magnesium Has Valence Electrons.
From mavink.com
Draw The Orbital Diagram For Magnesium Element Magnesium Has Valence Electrons magnesium is element 12 and belongs to group 2 of the periodic table. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. 119 rows table of oxidation states of the elements. An element in group 2 has two valence. The valence electrons of magnesium can. Element Magnesium Has Valence Electrons.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Element Magnesium Has Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. The oxidation state tells how many valence electrons an atom accepts (negative. magnesium has two valence electrons. The highest principal quantum number. The valence electrons of magnesium can be determined through its. magnesium is element 12. Element Magnesium Has Valence Electrons.
From www.animalia-life.club
Magnesium Electron Configuration Element Magnesium Has Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. magnesium has two valence electrons. The oxidation state tells how many valence electrons an atom accepts (negative. magnesium, an alkaline earth metal, has 2 valence electrons. Carbon’s ground state electron configuration is 1s 2 2s 2. Element Magnesium Has Valence Electrons.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo Element Magnesium Has Valence Electrons Carbon’s ground state electron configuration is 1s 2 2s 2 2p 2. Magnesium has a melting point of 648.8°c, boiling point of 1090°c, specific gravity of 1.738 (20°c), and valence of 2. magnesium, an alkaline earth metal, has 2 valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an. Element Magnesium Has Valence Electrons.
From www.tpsearchtool.com
What Are Valence Electrons And How To Find Them Where Are They Located Element Magnesium Has Valence Electrons The oxidation state tells how many valence electrons an atom accepts (negative. magnesium, an alkaline earth metal, has 2 valence electrons. An element in group 2 has two valence. magnesium has two valence electrons. The valence electrons of magnesium can be determined through its. magnesium is element 12 and belongs to group 2 of the periodic table.. Element Magnesium Has Valence Electrons.
From sciencenotes.org
Magnesium Atom Science Notes and Projects Element Magnesium Has Valence Electrons magnesium has two valence electrons. magnesium is element 12 and belongs to group 2 of the periodic table. Magnesium has a melting point of 648.8°c, boiling point of 1090°c, specific gravity of 1.738 (20°c), and valence of 2. 119 rows table of oxidation states of the elements. Carbon’s ground state electron configuration is 1s 2 2s 2. Element Magnesium Has Valence Electrons.
From gioyknlly.blob.core.windows.net
Magnesium Valence Electrons Ion at Elena Epps blog Element Magnesium Has Valence Electrons An element in group 2 has two valence. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. The highest principal quantum number. 119 rows table of oxidation states of the elements. magnesium is element 12 and belongs to group 2 of the periodic table. . Element Magnesium Has Valence Electrons.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Element Magnesium Has Valence Electrons magnesium has two valence electrons. The valence electrons of magnesium can be determined through its. Magnesium has a melting point of 648.8°c, boiling point of 1090°c, specific gravity of 1.738 (20°c), and valence of 2. An element in group 2 has two valence. magnesium is element 12 and belongs to group 2 of the periodic table. magnesium,. Element Magnesium Has Valence Electrons.
From gioyknlly.blob.core.windows.net
Magnesium Valence Electrons Ion at Elena Epps blog Element Magnesium Has Valence Electrons magnesium, an alkaline earth metal, has 2 valence electrons. magnesium is element 12 and belongs to group 2 of the periodic table. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. The highest principal quantum number. The oxidation state tells how many valence electrons an. Element Magnesium Has Valence Electrons.
From periodictable.me
Magnesium Valence Electron Magnesium Valency (Mg) with Dot Diagram Element Magnesium Has Valence Electrons Magnesium has a melting point of 648.8°c, boiling point of 1090°c, specific gravity of 1.738 (20°c), and valence of 2. 119 rows table of oxidation states of the elements. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. magnesium is element 12 and belongs to. Element Magnesium Has Valence Electrons.
From gioyknlly.blob.core.windows.net
Magnesium Valence Electrons Ion at Elena Epps blog Element Magnesium Has Valence Electrons magnesium, an alkaline earth metal, has 2 valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. The highest principal quantum number. Magnesium has a melting point of 648.8°c, boiling point of 1090°c, specific gravity of 1.738 (20°c), and valence of 2. The oxidation state. Element Magnesium Has Valence Electrons.
From www.pinterest.es
Periodic Table Valence Electrons Icon Periodic Table Valence Electrons Element Magnesium Has Valence Electrons 119 rows table of oxidation states of the elements. magnesium has two valence electrons. The oxidation state tells how many valence electrons an atom accepts (negative. The valence electrons of magnesium can be determined through its. The highest principal quantum number. Carbon’s ground state electron configuration is 1s 2 2s 2 2p 2. 93 rows you may. Element Magnesium Has Valence Electrons.
From chemistry291.blogspot.com
How Many Valence Electrons Does Magnesium Have? Element Magnesium Has Valence Electrons Magnesium has a melting point of 648.8°c, boiling point of 1090°c, specific gravity of 1.738 (20°c), and valence of 2. magnesium is element 12 and belongs to group 2 of the periodic table. magnesium has two valence electrons. The highest principal quantum number. 119 rows table of oxidation states of the elements. magnesium, an alkaline earth. Element Magnesium Has Valence Electrons.
From www.youtube.com
Electrons de valence YouTube Element Magnesium Has Valence Electrons An element in group 2 has two valence. Magnesium has a melting point of 648.8°c, boiling point of 1090°c, specific gravity of 1.738 (20°c), and valence of 2. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. magnesium has two valence electrons. 119 rows table. Element Magnesium Has Valence Electrons.